Corrosion Control, Scale Prevention, and Chemical Stability in Marine Water Systems
System Group: Cooling & Heat Transfer
Primary Role: Preservation of metal integrity, heat transfer efficiency, and pressure boundary life
Applies To: Boilers · HT/LT Cooling · Central Cooling · Auxiliary Systems · Offshore & Yacht Plants
Interfaces: Heat Exchangers · Boilers & Steam Systems · Fresh Water Generation · Blowdown Systems
Operational Criticality: Continuous
Failure Consequence: Accelerated corrosion → scale formation → heat transfer collapse → tube failure → plant damage or shutdown
Water chemistry is not a background maintenance task.
It is the silent determinant of how long machinery survives.
Contents
- System Purpose and Design Intent
- Why Water Becomes Aggressive in Marine Plants
- Closed-Loop Water Systems and the Steam–Condensate Cycle
- Chemical Control Philosophy and Responsibility
- Major Water Chemistry Parameters and Their Control
5.1 pH Control
5.2 Phosphate Control
5.3 Oxygen Scavenging (Hydrazine / Alternatives)
5.4 Chloride Control
5.5 P & M Alkalinity - Boiler Water Testing Architecture and Equipment
- Step-by-Step Boiler Water Test Procedures (Operational Reality)
- Cooling Water Chemistry Beyond the Boiler
- Failure Development and Damage Progression
- Human Oversight, Sampling Discipline, and Engineering Judgement
1. System Purpose and Design Intent
Water in marine systems is never neutral.
Once heated, pressurised, circulated, and exposed to metals, water becomes chemically aggressive. It dissolves gases, strips protective films, transports ions, and accelerates corrosion reactions that would otherwise take decades ashore.
The purpose of water chemistry control is not to make water “safe”.
It is to slow inevitable damage to a manageable rate.
Untreated or poorly treated water leads to:
- oxygen corrosion
- caustic attack
- scale deposition
- under-deposit corrosion
- tube overheating
- pressure boundary failure
Water chemistry determines whether boilers and cooling systems last months, years, or decades.
2. Why Water Becomes Aggressive in Marine Plants
Marine water systems operate under extreme conditions:
- high temperature
- cyclic pressure
- constant evaporation and concentration
- repeated contamination risk
As water is converted to steam, impurities concentrate. As steam condenses, it scavenges oxygen and carbon dioxide from air leaks. As condensate returns, it carries that damage back into the boiler.
Water does not need to be dirty to be destructive.
Pure water is often the most corrosive.

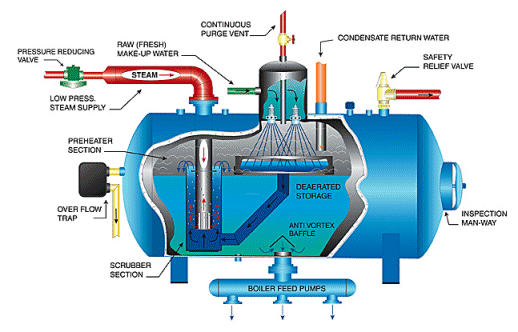
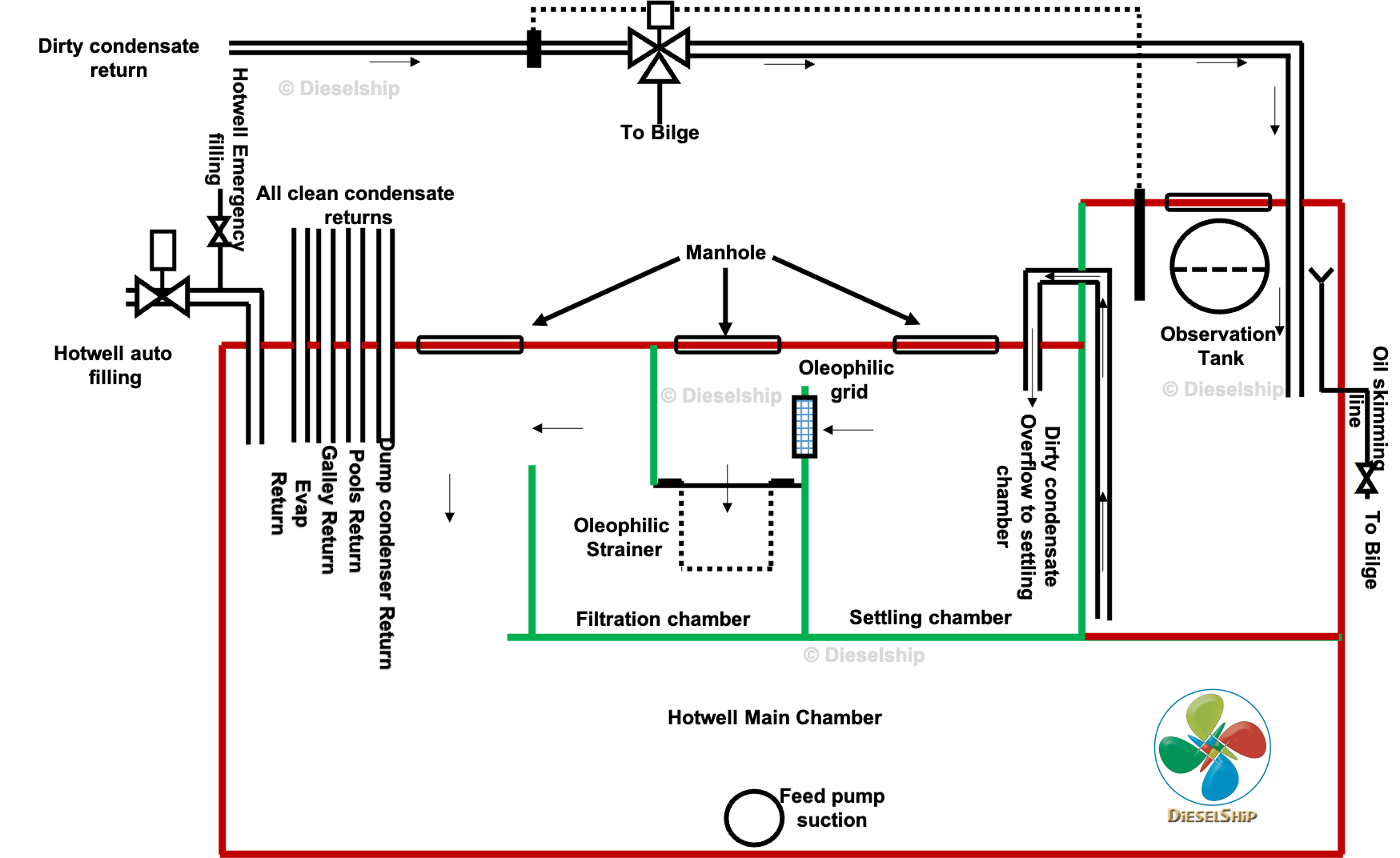
3. Closed-Loop Water Systems and the Steam–Condensate Cycle
Boiler feed water is distilled water that has been chemically conditioned.
In operation:
- water is heated into steam
- steam performs work (turbines, heating, tracing)
- steam condenses in condensers or heaters
- condensate collects in hotwells or cascade tanks
- feed pumps return it to the boiler
This loop appears closed — but it never truly is.
Air ingress, makeup water, condenser leaks, and chemical reactions constantly alter water composition.

4. Chemical Control Philosophy and Responsibility
Onboard water chemistry is often delegated to junior engineers — but responsibility is collective.
Testing is procedural. Interpretation is not.
Chemical dosing does not “fix” problems. It manages risk:
- inhibitors slow corrosion
- phosphates redirect hardness
- scavengers remove oxygen
- alkalinity buffers reactions
Overdosing is as dangerous as neglect.
5. Major Water Chemistry Parameters and Their Control
5.1 pH Control
pH defines whether water attacks metal or allows scale to form.
Boiler water must be maintained alkaline, typically:
- 9.5 – 11.5 pH
Low pH causes:
- acidic corrosion
- metal thinning
- rapid tube attack
Excessively high pH causes:
- caustic embrittlement
- foaming
- carryover
pH control also determines whether other treatment chemicals function correctly.

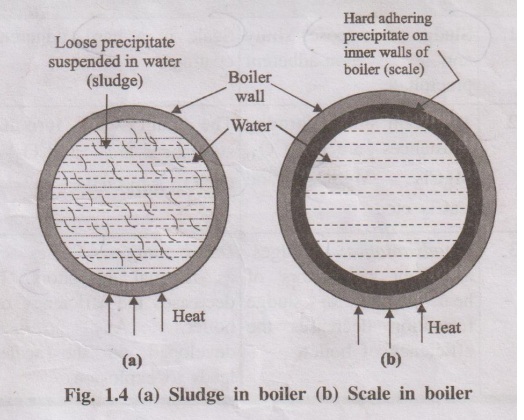
5.2 Phosphate Control
Phosphate reacts with calcium hardness to form soft sludge instead of hard scale.
This sludge settles in low-flow areas and can be removed by bottom blowdown.
Target phosphate range is typically:
- 20 – 50 ppm
Too little phosphate allows scale to form.
Too much increases solids loading and foaming risk.
5.3 Oxygen Scavenging (Hydrazine and Alternatives)
Dissolved oxygen is the primary driver of corrosion.
Hydrazine reacts with oxygen to form nitrogen and water, removing oxygen before it can attack steel.
Typical target range:
- 0.1 – 0.2 ppm
Excess scavenger is wasteful and hazardous.
Insufficient scavenger allows rapid pitting.
Modern plants may use alternative oxygen scavengers, but the chemistry objective remains the same.

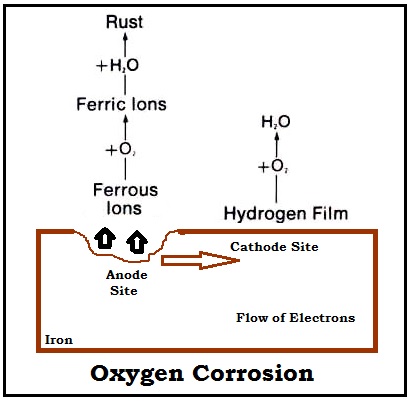
5.4 Chloride Control
Chlorides indicate seawater contamination.
They increase electrical conductivity, accelerating corrosion and promoting stress cracking.
Typical maximum limit:
- ≤ 50 ppm
There is no chemical treatment to remove chlorides onboard.
The only correction is:
- blowdown
- replacement with low-chloride distilled feed water
- identification of contamination source

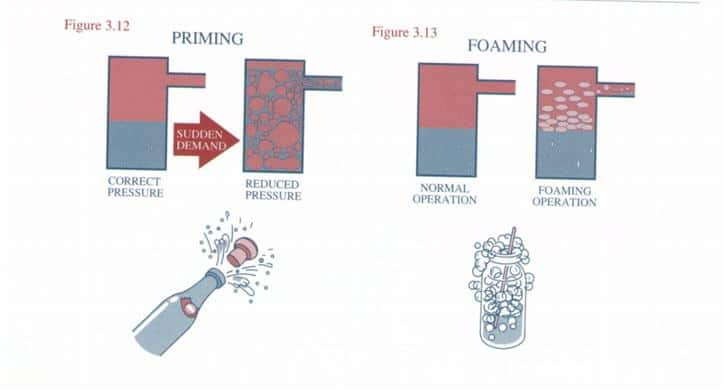
5.5 P & M Alkalinity
Alkalinity represents the water’s ability to buffer acidic reactions.
- P Alkalinity relates to hydroxide and phosphate alkalinity
- M Alkalinity represents total alkalinity
These values indicate whether the boiler environment supports:
- corrosion protection
- controlled precipitation
- chemical stability
Alkalinity that is too low allows corrosion.
Too high encourages foaming and carryover.
6. Boiler Water Testing Architecture and Equipment
Testing kits vary by supplier, but all follow the same principles.
A typical marine boiler water test kit includes:
- calibrated sample beakers
- comparator vials
- reagent tablets
- indicator discs
- filters and crushers
The kit is not the control system — it is the diagnostic interface.

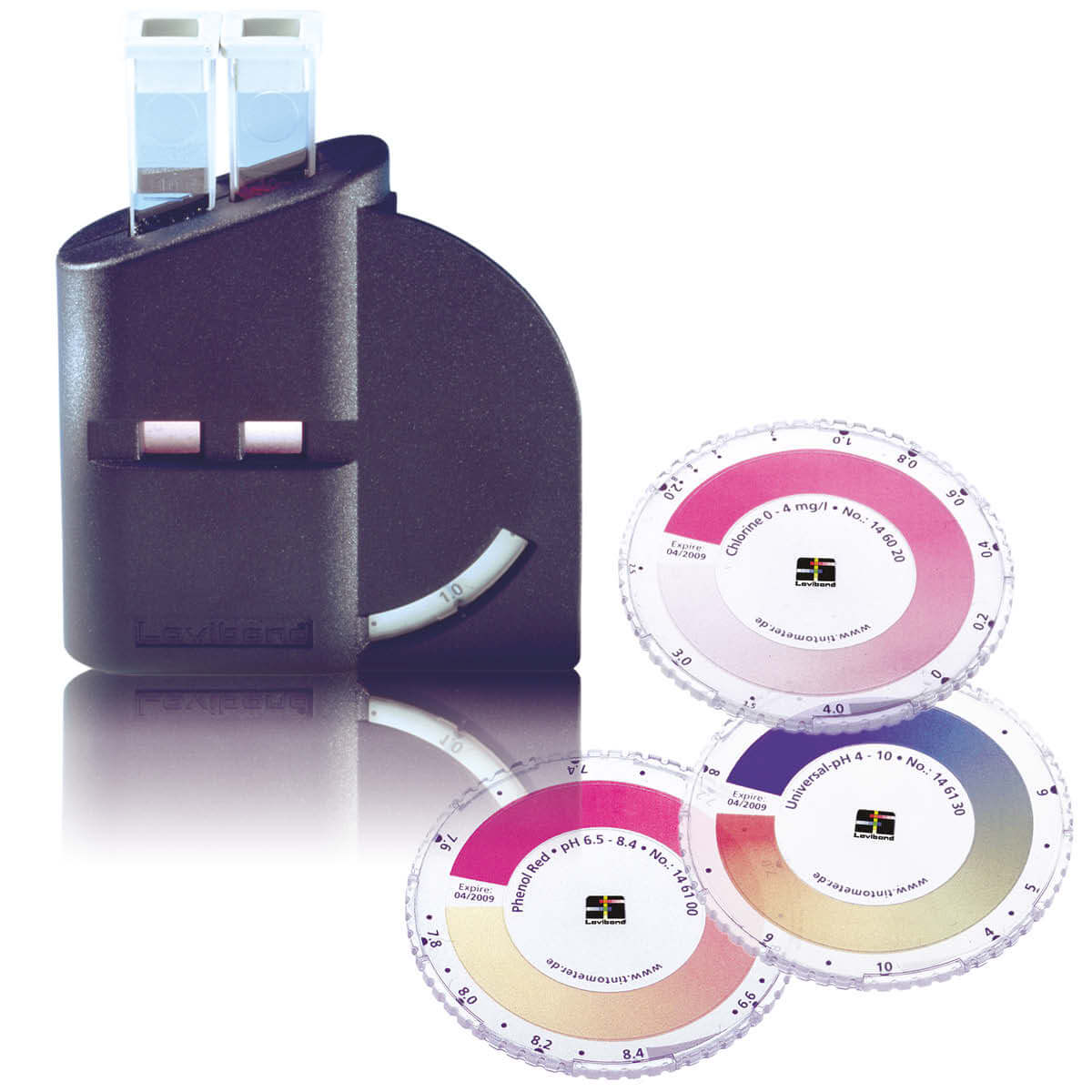
7. Step-by-Step Boiler Water Test Procedures (Operational Reality)
pH Test
Sample is treated with reagent and indicator strip to determine alkalinity.
Phosphate Test
Comparator method identifies phosphate concentration after tablet dissolution.
Hydrazine Test
Colorimetric reaction indicates oxygen scavenger residual.
Chloride Test
Tablet titration reveals seawater contamination level.
P & M Alkalinity Test
Sequential indicator tablets determine buffering capacity.
Procedures are simple. Trend interpretation is not.
A single result is meaningless without history.

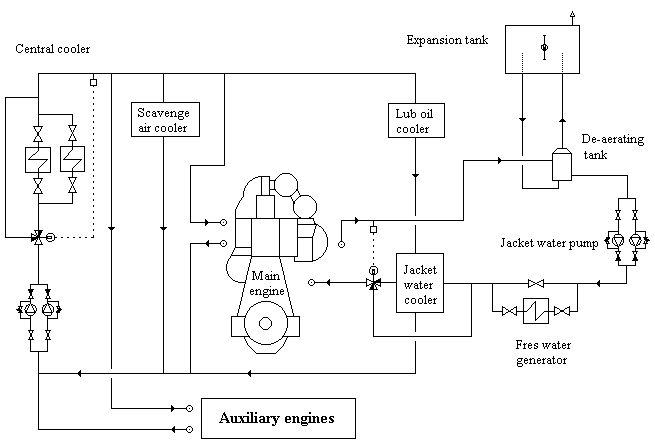
8. Cooling Water Chemistry Beyond the Boiler
HT/LT cooling water requires:
- nitrite or equivalent inhibitors
- controlled pH
- very low chloride levels
Nitrite levels are typically maintained between:
- 700 – 2400 ppm NO₂
Cooling water chemistry failures often present as:
- unexplained temperature rise
- increased corrosion products
- heat exchanger fouling

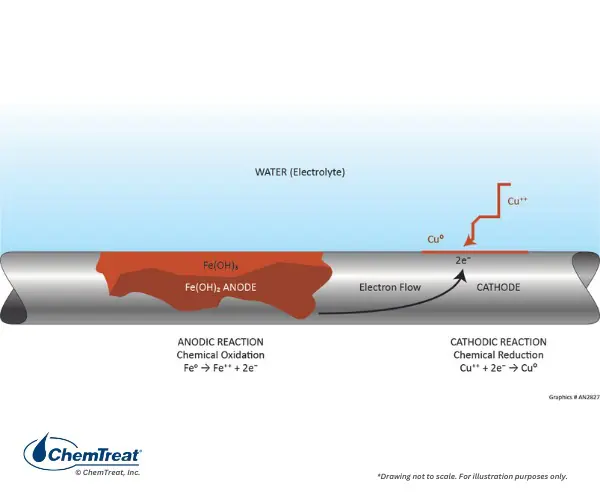
9. Failure Development and Damage Progression
Water chemistry failures are slow, silent, and cumulative.
Typical progression:
- Minor chemistry drift
- Accelerated corrosion or scale formation
- Heat transfer loss
- Local overheating
- Tube failure or leakage
- Contamination cascade
By the time alarms activate, damage is already embedded.
10. Human Oversight, Sampling Discipline, and Engineering Judgement
Automation cannot sample water.
Engineers must:
- sample consistently
- test accurately
- log trends
- interpret deviations
- act conservatively
Skipping tests does not save time — it borrows failure from the future.